- Submission process
- Forms and documentation
Submission
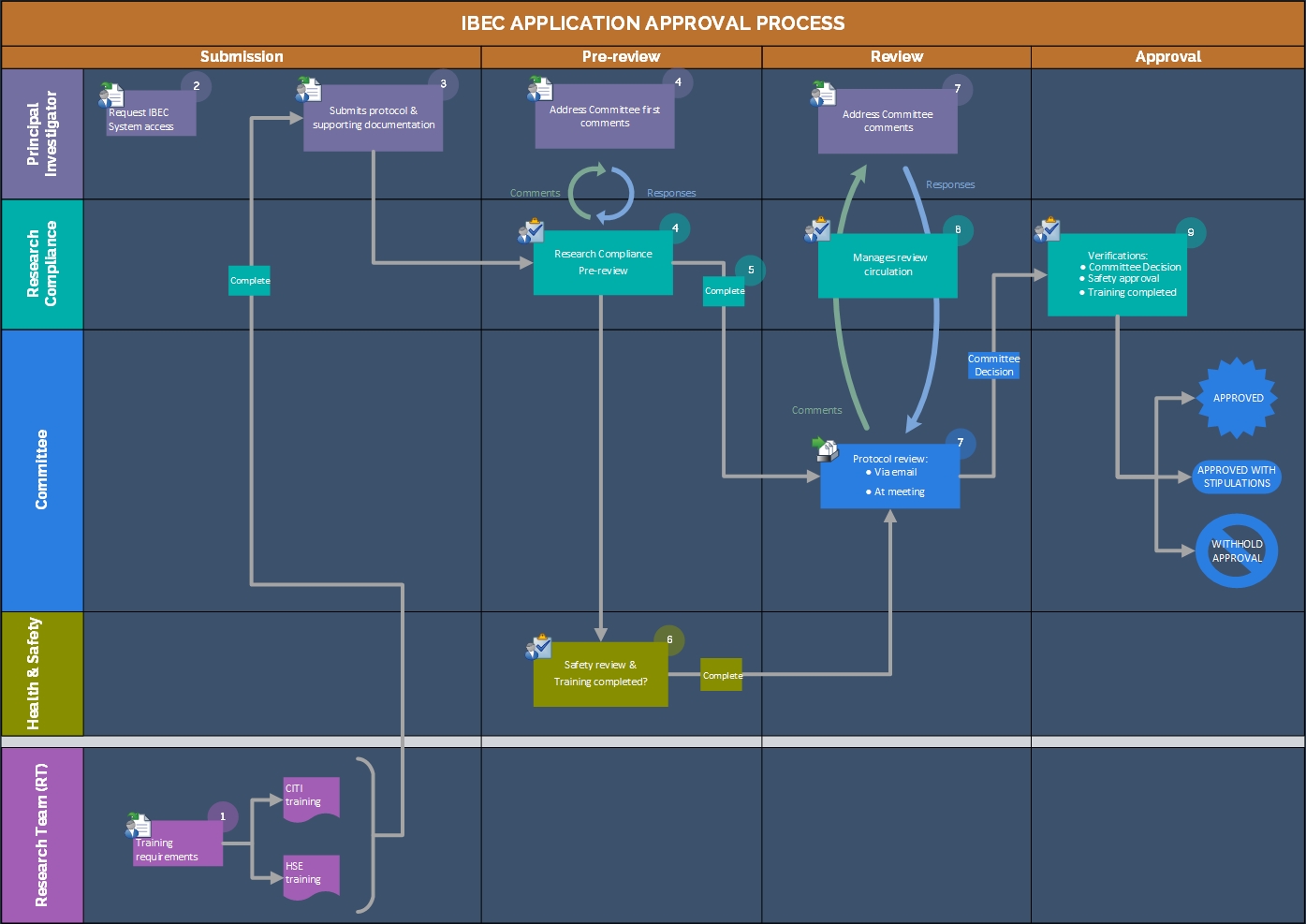
- All research team members complete required training: Health, Safety and Environment (HSE) and Collaborative Institutional Training Initiative (CITI) training.
- The principal investigator (PI) request the IBEC System Access by emailing ibec@kaust.edu.sa.
- The principal investigator (PI) submits the IBEC protocol and includes supporting documentation (Certification of Ethics Committee Approval Form, informed consent form, etc.). Please refer to the User Manual for any questions regarding the submission process.
- Research Compliance checks the IBEC protocol for completeness and shares comments on the protocol with the PI and discusses possible changes; the PI then submits all necessary revisions. RC verifies that pre-review is complete.
- After the pre-review is completed, Research Compliance forwards the IBEC protocol to the full Committee for review.
- Research Safety (HSE) conducts a safety assessment to ensure that all necessary laboratory safety practices vaccination and safety training are in place; Research Safety (HSE) notifies the IBEC when all safety requirements have been met.
- IBEC reviews the IBEC protocol, and sends any comments for the PI to Research Compliance.
Review methods:
• Circulation – Protocol reviewed by electronic circulation with no requirement for a meeting.
• Meeting - Protocol reviewed by electronic circulation, and must be discussed and approved at a convened meeting.
- Research Compliance manages review circulation. The PI submits a response to any comments along with a revised IBEC protocol addressing the comments.
- Once the IBEC protocol is approved, the PI receives the approval email.
Note: Approval will not be released by Research Compliance until training and safety requirements are completed.
Forms and Documentation
IBEC Protocol
IBEC System User Manual
Informed Consent Template
Waiver of Informed Consent Document
Collaborator Certification of Ethics Committee Approval
HSE Research Safety Information
Biosafety in the Laboratory
Hepatitis B Vaccination
HSE Biosafety Training
