- Submission
- Typical Review Timeline
- External Animal Study/Animal Tissue
- Forms
Submission
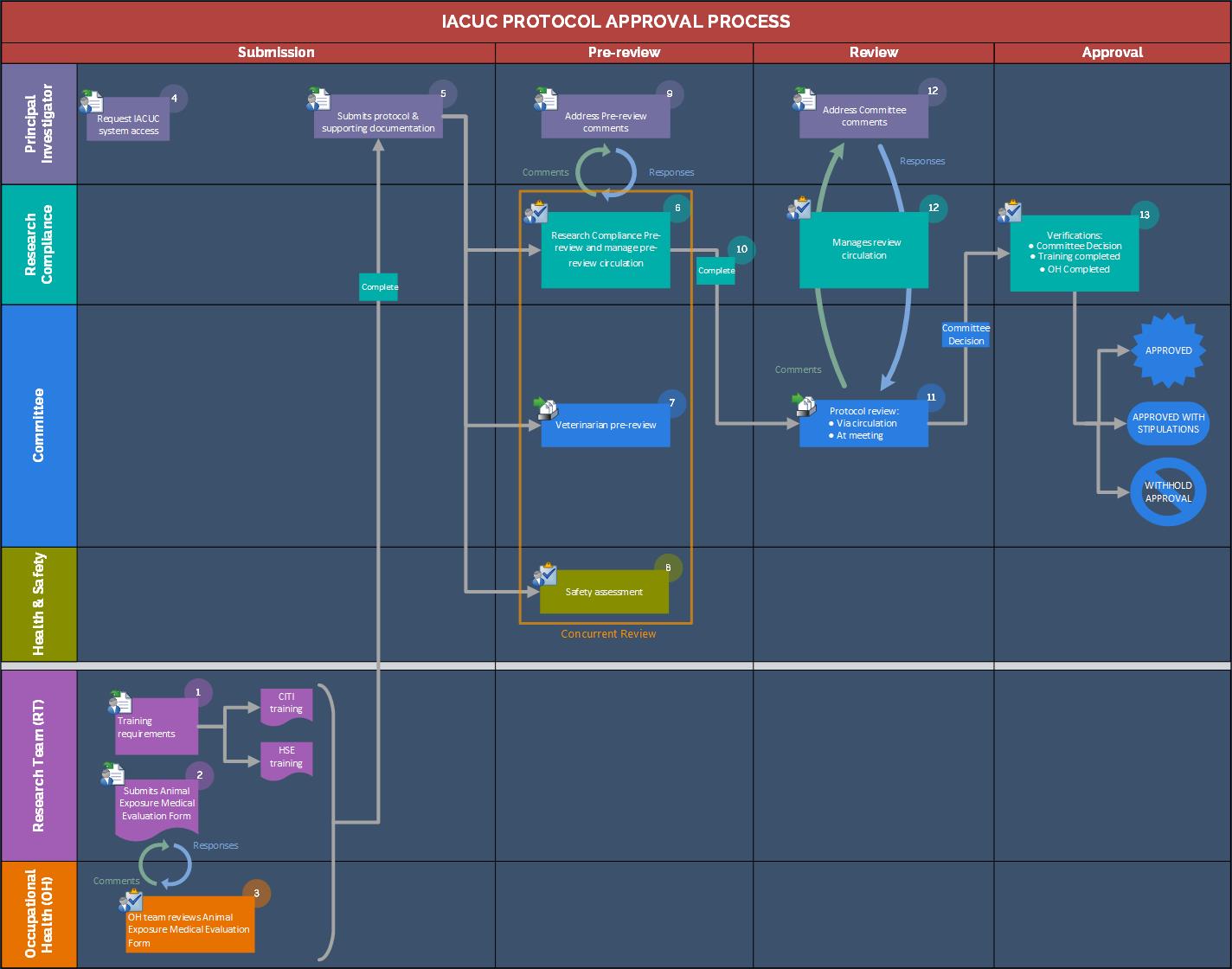
- All members of the research team complete required training: Health, Safety and Environment (HSE) and Collaborative Institutional Training Initiative (CITI) training.
- All members of the research team complete the Animal Exposure Medical Evaluation (AEME) on Salute.
- The KAUST Occupational Health team reviews the AEME to identify any medical prerequisites required for the team member to work with animals.
- The Principal Investigator (PI) request access to the IACUC Protal by emailing iacuc@kaust.edu.sa
- The PI submits the IACUC protocol and includes supporting documentation (Collaborator Approval, etc.). Please reference the IACUC User Manual - Rodents for rodent projects when completing the protocol. Please refer to the User Manual for any questions regarding the submission process.
- Research Compliance (RC) checks the IACUC protocol for completeness and adds any comments and/or suggestions to the protocol.
- The Veterinarian reviews the protocol and adds any comments and/or suggestions to the protocol.
- The HSE representative reviews the protocol, conducts a safety assessment to ensure that all necessary laboratory safety practices and safety training are in place, and adds any comments and/or suggestions to the protocol. The representative notifies IACUC when all safety requirements are met.
- The PI then submits all necessary revisions.
- After the pre-review is completed, RC forwards the IACUC protocol to the full Committee to evaluate compliance with regulations, analyze the harm-benefit of the animal study.
- IACUC reviews the protocol, and sends any comments for the PI to RC.
Review methods:
• Circulation – Protocol reviewed by electronic circulation with no requirement for a meeting.
• Meeting - Protocol reviewed by electronic circulation, and must be discussed and approved at a convened meeting.
- RC manages review circulation. The PI submits a response to any comments along with a revised protocol addressing the comments.
- Once the IACUC protocol is approved, The PI receives the letter of approval via email.
Note: Approval will not be released by Research Compliance until training, safety requirements, and occupational health (OH) evaluations are completed.
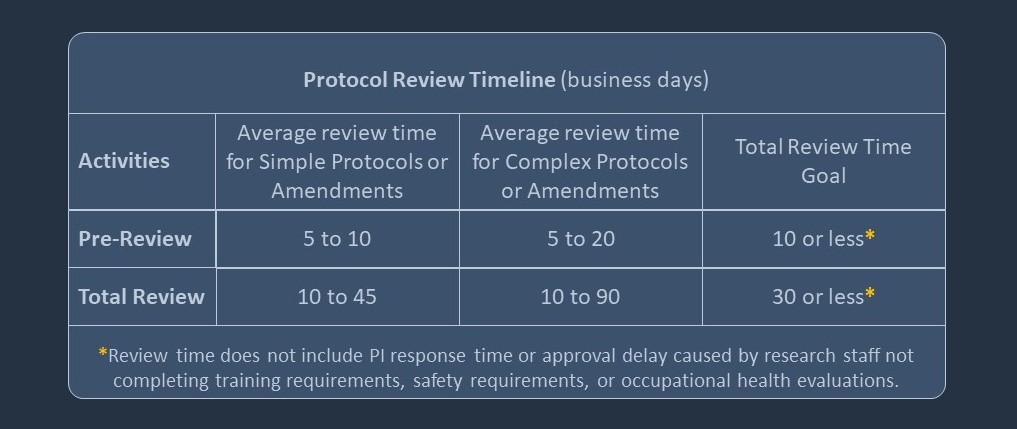
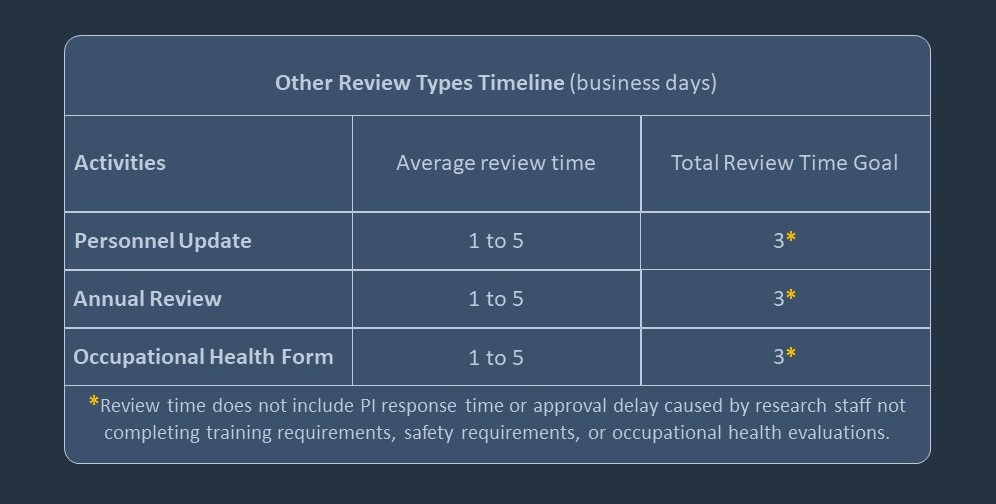
Typical Review Timeline
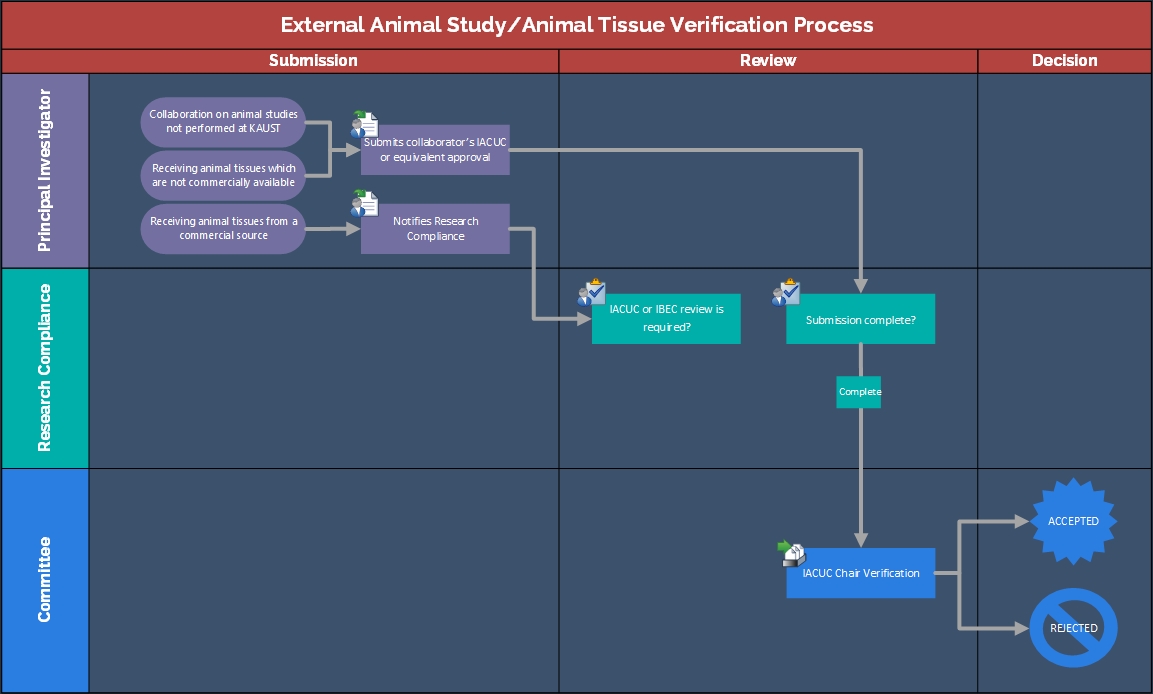
External Animal Study/Animal Tissue
IACUC Protocol
IACUC Protocol Form
IACUC Portal User Manual
Housing in PI Laboratory
Aquatic Animal Log exemple.pdf
Aquatic Animal Log exemple.xlsx
Terrestrial Animal Log exemple.pdf
Terrestrial Animal Log exemple.xlsx
Scoring Sheet Templates
General Scoring sheet.pdf
General Scoring sheet.xlsx
Body Condition Scoring sheet.pdf
Body Condition Scoring sheet.xlsx
Animal Irradiation Scoring sheet.pdf
Animal Irradiation Scoring sheet.xlsx



